

 Research & Development
Research & Development
R&DPOWERED BYSTRUCTURE-GUIDEDDRUG DESIGN
Targeting the disease at the active site
We pursue challenging diseases and design molecules with expert chemistry knowledge and multidisciplinary collaboration to help change how complement-mediated and other rare diseases progress - and how a patient feels.
We are world leaders in structure-guided drug design—succeeding in diseases where many others have failed—made possible by the integrated application of traditional biology and medicinal chemistry to design new molecules that are potent, selective, and bioavailable.
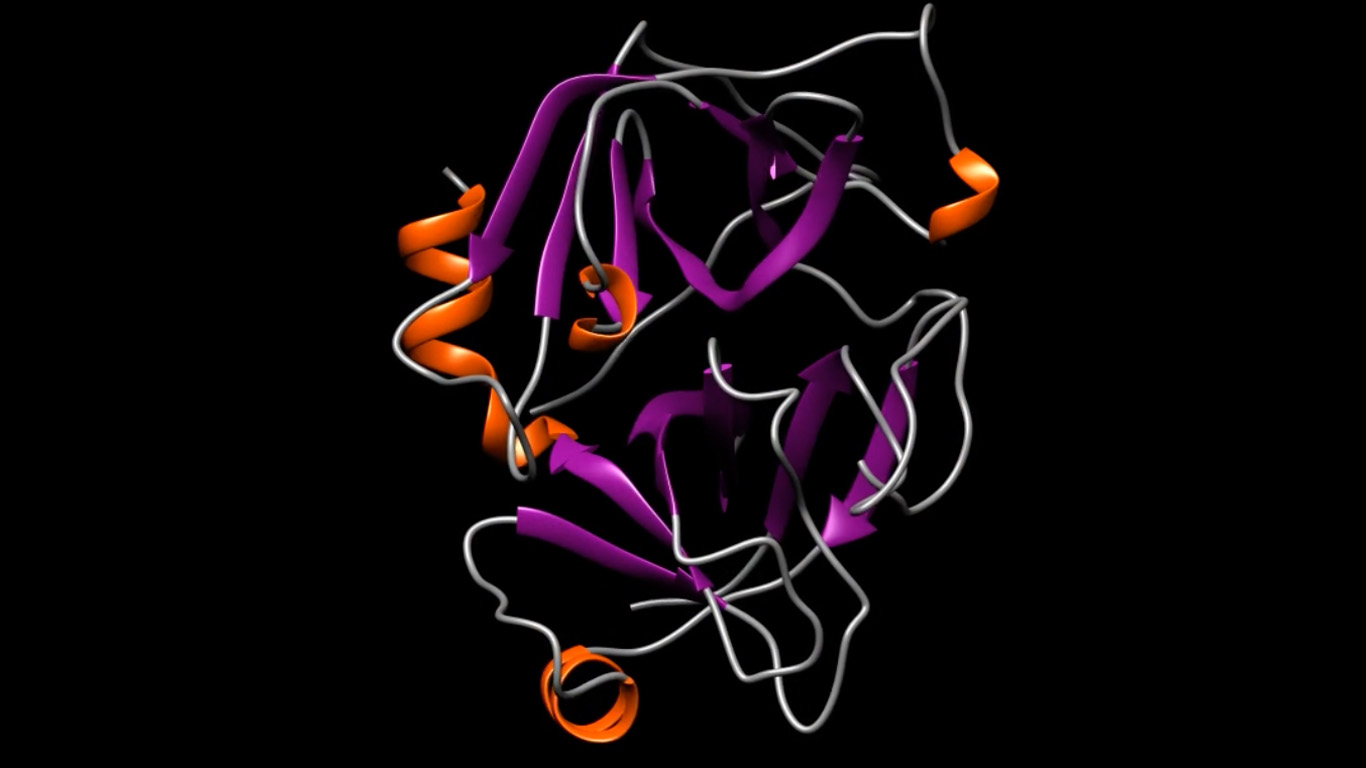

We leverage our experience in structural biology to identify the target protein in advance, and by determining the molecular structure of the protein, our scientists can design an optimal drug that uniquely fits a cell or binding site.
Structure-guided drug design requires an array of advanced technologies and computational tools, including X-ray crystallography, cryogenic electron microscopy, computer modeling of molecular structures, virtual screening, molecular docking, molecular dynamics simulations, and protein chemistry to focus on the 3-dimensional (3-D) structure of the active site of the target protein.
By identifying a target protein that plays a key role in a given disease, and by determining its 3-D structure, our scientists design highly potent, specific, and bioavailable drug molecules. BioCryst scientists design and synthesize drug candidates, atom by atom, to fit the active site on the protein, thereby suppressing its biological activity. The initial targets for structure-guided drug design are selected based on their involvement in the biological pathways integral to the course of a disease.
A growing pipeline of oral small-molecule and protein therapeutics for complement-mediated and other rare diseases
BioCryst development programs represent the potential to improve the well-being of people whose lives are currently limited by complement-mediated and other rare diseases. We discover novel, oral small-molecule and protein therapeutics that treat diseases in which significant unmet medical needs exist. An enzyme plays a key role in the biological pathway of the disease. Discover more >>
Complement-mediated diseases
The complement system is part of the body’s natural immune system and is responsible for helping the body eliminate microbes and damaged cells. It is composed of proteins that are primarily produced in the liver and circulate in the blood. Once activated, the complement system stimulates inflammation, phagocytosis, and cell lysis. Excessive or uncontrolled activation of the complement system can cause severe, and potentially fatal, immune and inflammatory disorders.
The complement system comprises biological cascades of amplifying enzyme cleavages involving more than 30 proteins and protein fragments and may be activated through three pathways1:
- Classical pathway (initiated by antibody-antigen complexes)
- Lectin pathway (initiated by microbial surfaces)
- Alternative pathway (constitutively active)
All three of these pathways share a common terminal pathway that culminates in formation of the cytolytic membrane attack complex.1,2 The alternative pathway also provides a critical amplification loop for all three pathways, regardless of the initiating mechanism.2
Helping people living with complement-mediated and other rare diseases
Using a structure-guided drug design process, we are developing first-in-class or best-in-class oral small-molecule and protein therapeutics that have the potential to transform patients’ lives.
1 in
50,000
people have a
DIAGNOSIS OF HAE
Hereditary angioedema (HAE)
HAE is a potentially life-threatening rare disease caused by a genetic deficiency of a protein called C1 esterase inhibitor (C1-INH). C1-INH plays an important role in preventing the bradykinin-forming system from becoming hyperactive and mediating swelling in HAE. Hereditary angioedema is a rare condition, affecting between approximately 1 in 10,000 to 1 in 50,000 people.3 Left untreated, patients with HAE often have multiple attacks every month, and the swelling from each attack can last for 2 to 4 days.4
References
1. Barratt J, Weitz I. Complement factor D as a strategic target for regulating the alternative complement pathway. Front Immunol. 2021;12:712572.
2. Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I: molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262.
3. Bernstein JA. HAE update: epidemiology and burden of disease. Allergy Asthma Proc. 2013;34(1):3-6.
4. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24:S292-S298.
 Italy
Italy  Japan
Japan  Spain
Spain 